Skeletalis Raises $8M for Osteoporosis Treatment
Small-molecule delivery platform enables more specific intervention in bone disease progression
BOSTON, November 17, 2025 — Skeletalis, a drug development company focused on the next generation of therapies for treating musculoskeletal diseases, today announced that it has raised $8 million to advance its osteoporosis small molecule candidate. Pillar VC led the round, with support from KdT Ventures, age1, and Slocum Management.
“Osteoporosis treatment is ripe for innovation,” said Ben Swanson, DDS, PhD, founder and CEO of Skeletalis. “Current therapies are limited by serious side effects that compromise safety, adherence, and long-term efficacy. At Skeletalis, we’re developing precision medicine with tissue and cell specificity, enabling more targeted intervention in osteoporosis progression.”
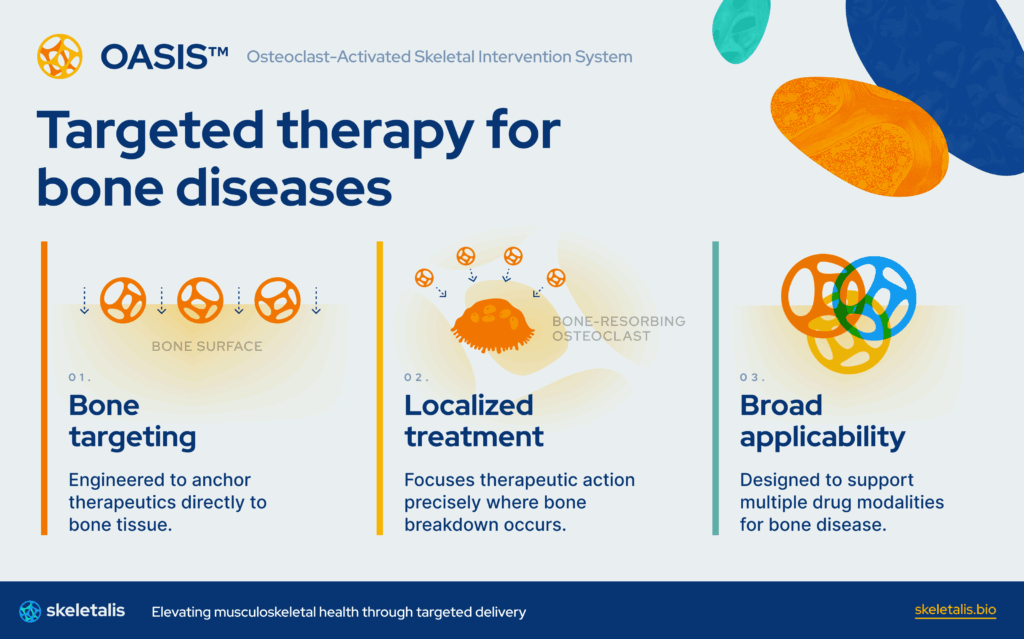
Osteoporosis affects over 10 million U.S. adults, over two thirds of whom are post-menopausal women. The disease causes fragile bones, debilitating fractures, and more than $50 billion in U.S. healthcare system costs. For decades, the standard of care in osteoporosis has asked patients to trade efficacy for safety. These therapies slow bone loss but carry rare yet devastating bone safety concerns, poor adherence, and diminishing returns. Recent regulatory reforms recognizing the urgent need for preventive, accessible bone health solutions have opened the door for innovation that finally meets patients where they are. Skeletalis is leading this new era with its bone-targeted technology, designed to deliver potent, localized disease-modulating therapy directly to the skeleton while minimizing systemic exposure. By re-engineering how osteoporosis drugs engage their target, Skeletalis aims to extend therapeutic durability, improve tolerability, and transform chronic bone care from a compromise into a true resolution of skeletal health.
“Skeletalis is developing a therapy that has the potential to finally offer patients a treatment option that does not come with a major concession,” said Thomas de Vlaam, Partner at Pillar VC. “The need for better treatments has been apparent for a long time, and while complex regulatory requirements previously prohibited new approaches, they have now shifted to become a tailwind for anyone trying to bring new medicines to patients. With the FDA’s landmark FNIH-ASBMR SABRE decision recognizing bone mineral density as a registrational endpoint for fracture prevention, the path is finally open for faster, smarter osteoporosis drug development.”
While Skeletalis is starting with osteoporosis, their core technology has first-line potential across degenerative bone diseases, from periodontitis to conditions affecting children. Skeletalis is led by Dr. Ben Swanson, a dentist-scientist whose research at the University of Michigan led to the creation of the core technology for bone-targeted therapeutics, alongside Dr. Colin Greineder, an associate professor of pharmacology and physician-scientist specializing in translational drug design. Supported by program leaders who advanced the prior generation of osteoporosis medicines, Skeletalis unites scientific rigor with a bold new vision for safer, more durable bone health solutions. To learn more about Skeletalis, including open positions, visit skeletalis.bio/#careers.
About Skeletalis
Skeletalis is developing the next generation of therapies for musculoskeletal diseases. The company’s first small molecule candidate is a first-in-class, bone-targeted osteoporosis treatment designed to prevent fractures and preserve bone mineral density while avoiding the safety liabilities that have constrained existing therapies. To learn more, visit skeletalis.bio.